| Top Priority |
|
|---|---|
| High Priority |
|
| Others |
|
Focus Area 2Compliance, Product Quality, and Safety
The Future We Envision
Our first priority: Continuous striving for product quality, safety and compliance to ensure sustainability and patient safety
As a globally leading MedTech company, our first priority is the provision of reliable, high-quality products. To further enhance the trusted reputation of Olympus products, services, and solutions, we are now implementing a comprehensive quality transformation program that will improve both our Quality Management System and our regulatory compliance.
Simultaneously, we will also continue to cultivate an environment of integrity and compliance throughout our organization, by maintaining and improving our Compliance Management System, which is designed to raise confidence and trust in Olympus.
- Materiality Topics
WhyWhy are we taking these actions?
To build and maintain trust in the company and its products
Olympus Perspective 1: Patient Focus—Demand for Quality and Reliability in Medical Devices
Medical devices that directly affect the lives and health outcomes of patients
As a medical device manufacturer, we primarily develop products—such as endoscopes and endo-therapy devices—that exceed customer requirements. Medical devices directly impact the lives and health outcomes of patients, and accordingly these products must be safe, reliable, and of the highest quality. Product recalls or suspensions of shipment, due to defects or other quality issues, can lead to delays in the treatment offered at clinical facilities. Accordingly, Olympus must continue to ensure product quality, based upon our principle of “Patient Focus.”
As medical technology becomes increasingly sophisticated, the laws and regulations enacted by nations worldwide to address medical devices are becoming more stringent each year. Therefore, it is essential that we maintain product quality while staying up-to-date on—and complying with—this evolving regulatory environment.
Olympus Perspective 2: Compliance is the Foundation of Trust
Building trust through fair and transparent business activities, in compliance with laws and regulations
Our company prioritizes compliance with applicable laws and regulations, recognizing it as fundamental to our success and sustainability. Always operating in a manner consistent with Our Corporate Philosophy, and upholding Our Core Value of Integrity, we are committed to maintaining trust with all stakeholders, including customers, patients, employees, shareholders, regulators, and other third parties.
Olympus has a zero-tolerance for bribery, corruption, or any other form of illegal, unlawful, or anti-competitive activity. This unwavering commitment safeguards our company’s reputation and mitigates legal risks. For example, when interacting with healthcare professionals we communicate appropriate product information in good faith, using only approved promotional materials that comply with applicable laws and regulations.
HowHow will we achieve this?
Fostering employee awareness while strengthening systems and structures
Approach 1: Patient Focus— Policy Development for Company-Wide Awareness
Redefining Our Core Values, Rethinking Company Strategy
In its new company strategy, announced in May 2023, the Olympus Group sets out “patient safety and sustainability” as one of its guiding principles. Furthermore, to foster a quality-focused corporate culture befitting of a global MedTech Leader, and clarify the values that are important to us, in February 2024 we added “Patient Focus” to the Core Values contained in our Corporate Philosophy.
In line with the Olympus Quality Policy, we are engaged in shaping an improved corporate culture that is more focused upon patient safety and security. This activity was initiated by our Quality Assurance and Regulatory Affairs division, but has developed into a company-wide priority shared by all employees.
〈Related Materiality Topics〉Quality and safety in product, service and solution
Approach 2: Reinforcement of System to Comply with Quality Laws and Regulations
Continuous improvement of the company’s internal quality management system, in both practice and supervision
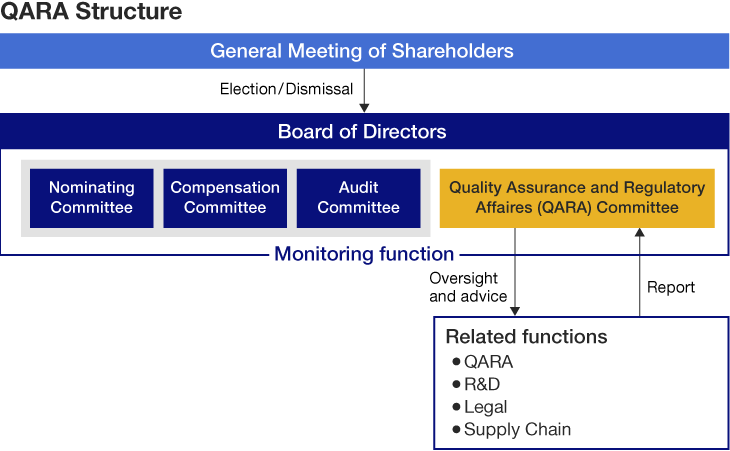
The Olympus Group has appointed a Chief Quality Officer (CQO) to oversee quality and regulatory function. This new role is responsible for reviewing and improving our working processes worldwide, in conjunction with Quality Assurance and Regulatory Affairs (QARA) members in each region.
We established a QA&RA Committee at the Board of Directors in April 2023. This is a voluntary committee composed of outside directors., Relevant functions including QARA; R&D; Legal; and Supply Chain, report to the committee on improvement progress. The committee oversees and provides advice for the development of global quality management systems, products, and services to meet the expectations of regulators while ensuring compliance with relevant laws and regulations.
〈Related Materiality Topics〉Quality and safety in product, service and solution / Risk and crisis management, risk culture
Approach 3: Transformational Initiatives for Patient Safety and Our Future Growth
Implementing Elevate: a holistic remediation and quality transformation program
As a global MedTech company, Olympus seeks to be recognized for the quality, value, and innovation that our people, products, and services bring to society each and every day. Towards this goal, we consistently demonstrate an unwavering commitment to quality and patient safety in everything we do.
Elevate, launched in fiscal year ended March 2024, is a multi-year quality transformation program that spans our entire company. This initiative embodies four key, long-term goals:
- Strengthening our patient safety focus and product quality culture
- Embedding sustainable, repeatable processes and compliance
- Fostering constructive relationships with health authorities
- Leveraging quality as a competitive advantage
Elevate involves teams from R&D, operations, supply chain, service, repair, and various regional organizations working together to improve global quality systems and processes, and standardize operations, in order to satisfy global regulatory requirements. The program will not only strengthen quality management systems—it will also become a key facilitator of innovation, growth, and increased profitability. These it will achieve through sustainable benefits such as improved lifecycle management, and digitally enabled processes to reduce costs, raise effectivity, and drive efficiency in products’ development, clearing, and launch timelines.
〈Related Materiality Topics〉Quality and safety in product, service and solution / Ethical marketing practice
Approach 4: Establishment of Global Rules
Our Global Code of Conduct: a foundational set of expectations, demonstrating our strong commitment to integrity
We expect every employee, manager, officer, and director to understand and comply with our Global Code of Conduct (Code), which ensures compliance with applicable laws and regulations while acting with integrity.
The Code was established to help affirm that Our Purpose and Core Values are embodied in the company’s daily operations. The Code affirms our unwavering commitment to integrity, reflecting the company’s high expectations, and enabling us to navigate complex business and regulatory environments while maintaining a focus on patients. Adherence to the Code, along with applicable global, regional, and local policies, ensures our business is conducted both ethically and responsibly.
〈Related Materiality Topics〉Business ethics and compliance
Approach 5: Compliance Management System
Initiating compliance activities in each region, under the supervision of the Global Chief Compliance Officer

Our Chief Executive Officer (CEO) bears the highest responsibility for compliance with applicable laws and regulations for the Olympus Group. The CEO has appointed a Global Chief Compliance Officer (CCO) with responsibility for the group’s Compliance Management System. The CCO delivers periodic reports on compliance activities to the Board of Directors and its Audit Committee, and these bodies consult with the CCO as necessary.
The CCO also chairs a Global Leadership Team (GLT), which consists of Regional Chief Compliance Officers, a Chief Privacy Officer, and other personnel designated by the CCO. Working with members of the GLT, the CCO ensures that relevant internal regulations are adhered to in regional business centers. The CCO and GLT also ensure compliance activities align with our Compliance Management System and the other management systems overseen by the CCO, reflecting best practices.
〈Related Materiality Topics〉Business ethics and compliance
WhatWhat specifically will we do?
Initiative Example 1:
Promoting Elevate, our comprehensive remediation and quality transformation program

To ensure patient safety while supporting growth, since the beginning of the fiscal year ending March 2024 Olympus has been implementing a comprehensive, multi-year remediation and quality transformation program named Elevate.

