Olympus Introduces EZ Shot 3 Plus 25 G EUS Needle with Enhanced Maneuverability for Uncompromised Access to Any Lesion, Consistent Performance to Potentially Reduce Procedural Costs and Procedure Time Entire EZ Shot 3 Plus Line-Up Now Cleared for Fine Needle Biopsy
September 14, 2018
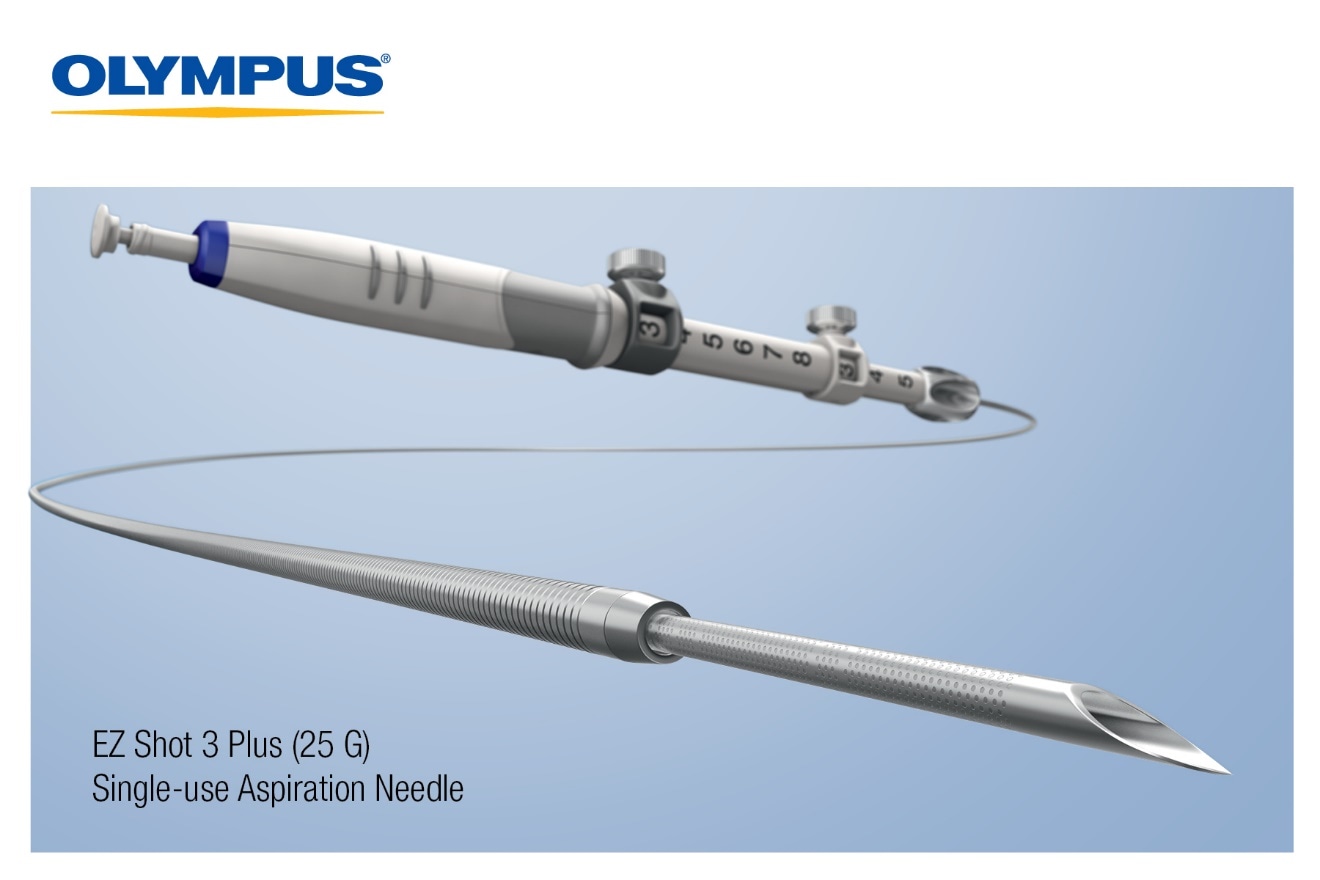
On September 14, 2018, Olympus,
a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today the FDA clearance of its EZ Shot 3 Plus 25 G needle as well as an expanded indication for the EZ Shot 3 Plus product line-up for both fine needle aspiration (FNA) and fine needle biopsy (FNB). The uncompromised access, enhanced puncturability, predictable trajectory and distinct echogenicity of the EZ Shot 3 Plus line, combined with the new 25 G offering and expanded indication for FNA and FNB, can drive improved staging of disease
Press releases are company announcements that are directed at the news media.
Information posted on this site is current and accurate only at the time of their original publication date, and may now be outdated or inaccurate.

