Olympus Announces Launch of EVIS X1™ Endoscopy System in ChinaThe product now launched in all of Olympus' major markets1
November 6, 2023
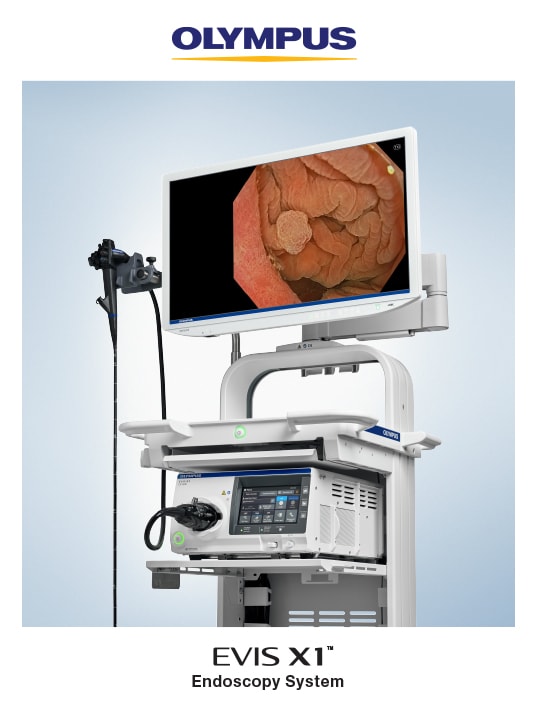
TOKYO, November 6, 2023 – Olympus Corporation (Olympus), a global medical technology company committed to making people’s lives healthier, safer and more fulfilling, announced today the launch of its next-generation EVIS X1™ endoscopy system in China. This marks the availability of the EVIS X1 endoscopy system in all of Olympus’ major markets.
EVIS X1 is Olympus’ most advanced endoscopy system. It was first released in Europe and certain regions in Asia in April 2020, followed by Japan in July 2020 and the U.S. in October 2023, and has now received clearance in China.
Olympus® GI endoscopy systems are used by physicians to help them to diagnose, treat, and observe diseases and disorders of the upper and lower GI tract such as acid reflux, ulcers, Crohn’s disease, Celiac disease, and colorectal cancer (CRC). An endoscope is commonly used during a screening colonoscopy, when a gastroenterologist examines the lining of the colon and may remove any potentially cancerous growths, known as polyps. The addition of new imaging technologies in the EVIS X1 endoscopy system may help physicians to visualize these abnormalities.
CRC represents the third most common cancer diagnosis for men and the second most common cancer diagnosis for women2. CRC is a preventable and treatable disease, however, with a 5-year survival rate of up to 91%, if caught at a localized stage3. Early discovery and diagnosis is a crucial step in preventing cancer.
The EVIS X1 endoscopy system will be demonstrated at the 6th annual China International Import Expo held in Shanghai from November 5-10.
For more information, visit the EVIS X1 endoscopy system page.
###
1 Japan, Europe, United States, China, and certain areas of Asia.
2 World Health Organization International Agency for Research on Cancer, 2020
3 World Health Organization International Agency for Research on Cancer, 2020
About Olympus
As a leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus' portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments. For more information, visit medical.olympusamerica.com.
* Products or devices presented include future technology which may be pending regional regulatory approval and are not available for sale in all regions.
* The contents in this website including products availability, specifications or prices are the information as of the date of announcement and are subject to change without prior notice.
* Information is intended to be presented to the media, shareholders, investors, and other interested parties. Information about our medical products (including products currently under development) included in this website is not intended for advertising or medical advice.
* Olympus Corporation assumes no responsibility for any damage resulting from the use of this material.
* All company names and product names mentioned in this website are trademarks or registered trademarks of their respective companies. ® and TM marks are not specified in this website. All trademarks and registered trademarks are the property of their respective owners.

