Olympus to Begin Sales Activities of Aplio i800 EUS
in Europe, Japan and OceaniaAdvancing the dimensions of endosonography
September 24, 2024

Aplio i800 EUS
TOKYO, September 24, 2024 - Olympus Corporation (Olympus), a global MedTech company committed to making people’s lives healthier, safer and more fulfilling, announces the beginning of sales activities for its new Aplio i800 diagnostic ultrasound system for Endoscopic Ultrasonography (EUS), with the device scheduled to be available for purchase starting in Europe from July 2024, followed by Japan and Oceania in the coming months. The processor – manufactured by Canon Medical Systems Corporation (Canon Medical) and distributed by Olympus – joins a comprehensive product portfolio of Olympus’ Ultrasound Endoscopes and EndoTherapy devices. It is the first output from the recently announced business alliance between the two global MedTech companies, which aims to provide the market with advanced EUS equipment capable of delivering high-quality diagnostic imaging.
The Aplio i800 EUS processor generates ultrasound images to support an accurate and reliable diagnosis of Hepato-Pancreato-Biliary conditions, including liver and pancreatic cancer. Diagnosing these diseases is particularly challenging in imaging today due to their complex anatomical locations, the need for high-resolution imaging to detect also early-stage abnormalities, and the need for precise differentiation between benign and malignant lesions. “I believe Aplio has opened the doors to a new era of patient care and research innovations in EUS”, says Prof. Dr. Pietro Fusaroli from the hospital S. Maria della Scaletta in Imola, Italy.
Innovative imaging technology supports rapid and reliable diagnosis and treatment
An EUS visualization benefit is inherent in the differentiation in shading it provides with the ultrasound image. D-THI (Differential Tissue Harmonic Imaging), developed by Canon Medical, enables high-resolution images with high resolution from near to far field. This expects rapid identification of tumors, cysts, and other lesions from normal tissue, supporting diagnosis and treatment. “With D-THI, endoscopists are assisted in identifying each of the five layers of the gastrointestinal wall with high contrast”, says Dr. Rodica Gincul from the Jean Mermoz Private Hospital – Ramsay Health in Lyon, France, while her colleague Dr. Bertrand Napoléon emphasizes: “The higher resolution imaging of Aplio at greater depth through the use of D-THI allows me to safely visualize and reach bile ducts even at very deep locations, improving therapeutic biliary drainage and patient care.
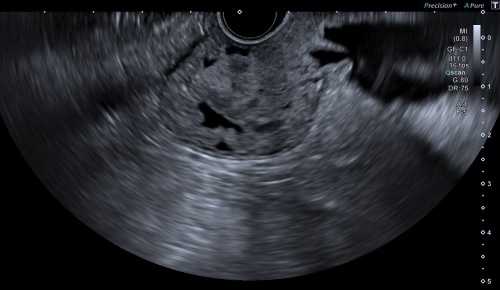
D-THI
Equipped with imaging functions that capture minute and slow blood flow, as well as other functions that contribute to highly reliable diagnosis.
A wide variety of EUS-supporting functions are provided to support reliable diagnosis and treatment decisions. SMI (Superb Micro-vascular Imaging), Canon Medical's proprietary technology, is a blood flow imaging technique that captures minute and slow blood flow. It supports safer procedures, even when performing puncture under ultrasound control, because it can depict low-velocity blood flow with high sensitivity, high resolution, high frame rate1, and low artifacts2, which may be difficult to depict with color Doppler3.4 “SMI allows detailed visualization of microvascular structures. With SMI, I feel more confident in my procedures.” says Dr. Khanh Do-Cong Pham from the Haukeland University Hospital in Bergen, Norway. SWE (Shear Wave Elastography) is a technology that displays tissue hardness as a numerical value or color map, enabling a more objective evaluation of hardness.
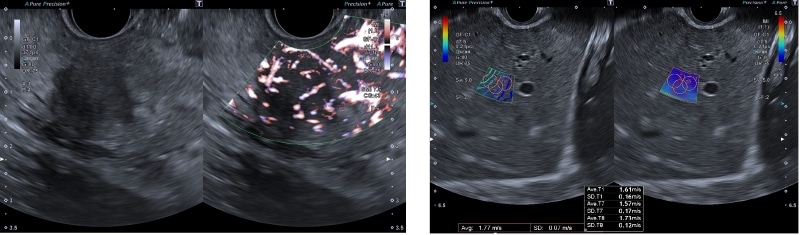
Left: SMI / Right: SWE
System operation can be customized according to user needs
The Aplio i800 system allows the height of the operation panel to be adjusted electrically at the touch of a button to suit the healthcare professional, contributing to improved operability and improved ergonomics during examinations. In addition, the buttons on the operation panel and touch panel can be positioned according to the user's needs, thereby improving usability.
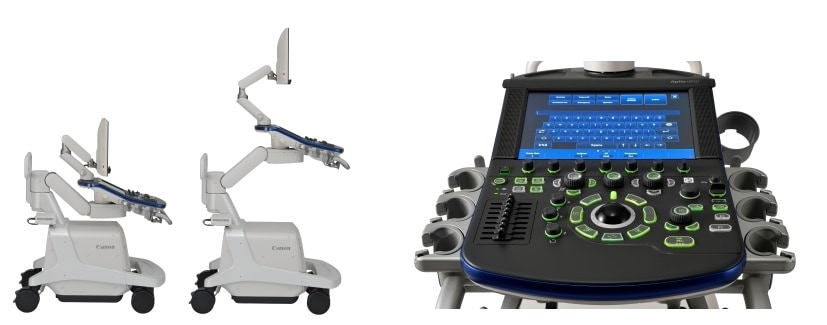
Left: Height adjustable to meet user needs / Right: Front view of operation panel/touch panel
DISCLAIMER
* The contents of this press release are aimed at healthcare professionals only.
* The healthcare professionals mentioned in this press release have a business relationship with Olympus as part of their contracted market preference test with Aplio i800 EUS
*Information on this news release does not replace the labeling or IFU for the devices and any user of the medical device must at all times observe all mandatory information, found, in particular, on the labels and the IFU.
*The products referenced on this news release may not be available in all jurisdictions or regions. In addition, indications for use may vary by jurisdiction or region. Please contact your Olympus representative if you have any questions about the availability of the products in your area.
1 The number of images that can be produced in one second. The higher the frame rate, the smoother the image.
2 Imaging artifacts are irregularities or distortions in the ultrasound image that are not true representations of the tissue, a low artifact rate is desirable.
3 A method of depicting blood flow in the human body and displaying it in real time.
4 Kim, DG., Lee, J.Y., Ahn, JH. et al. Quantitative ultrasound for non-invasive evaluation of subclinical rejection in renal transplantation. Eur Radiol 33, 2367–2377 (2023).
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide best-in-class solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit https://www.olympus-global.com/ and follow our global X account: @Olympus_Corp.
* Products or devices presented include future technology which may be pending regional regulatory approval and are not available for sale in all regions.
* The contents in this website including products availability, specifications or prices are the information as of the date of announcement and are subject to change without prior notice.
* Information is intended to be presented to the media, shareholders, investors, and other interested parties. Information about our medical products (including products currently under development) included in this website is not intended for advertising or medical advice.
* Olympus Corporation assumes no responsibility for any damage resulting from the use of this material.
* All company names and product names mentioned in this website are trademarks or registered trademarks of their respective companies. ® and TM marks are not specified in this website. All trademarks and registered trademarks are the property of their respective owners.

