Olympus Announces FDA Clearance of Its Most Advanced Imaging EndoscopesEDOF™ Technology Provides a Continuously Sharp Image with Minimal Focal Adjustment
as Part of the EVIS X1™ Endoscopy System
May 28, 2025
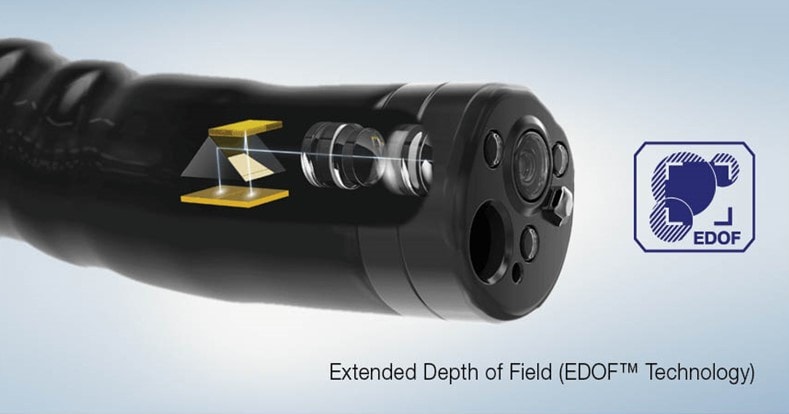
Extended Depth of Field (EDOF) Technology
CENTER VALLEY, Pa. (May 28, 2025) – Olympus Corporation of the Americas announced today FDA 510(k) clearance of its EZ1500 series endoscopes featuring Extended Depth of Field (EDOF™) technology.
EDOF™ endoscopes – the GIF-EZ1500 gastroscope and the CF-EZ1500DL/I colonoscope – represent Olympus’ most advanced imaging and latest innovation as part of the EVIS X1™ endoscopy system. By allowing physicians to obtain sharp images, EDOF technology allows an entire lesion to be kept in focus and may aid in detection as endoscopists inspect the mucosal lining of the GI tract.
EDOF technology creates an image in total focus by using two prisms to split light entering the endoscope lens into two separate beams with near- and far-focused images. Those beams are then projected simultaneously onto an image sensor, combining them into one image with a wide depth of field.1
Compared to the previous generation of Olympus® scopes, this technology provides improved visibility and less blurring. The EZ1500 series EDOF scopes allow for closer distance in normal mode compared to the CF-HQ190L/I – 3mm vs. 5mm – without blurring, limiting the need to switch from normal to near mode.
The GIF-EZ1500 gastroscope and the CF-EZ1500DL/I colonoscope also feature a lightweight ErgoGrip™ control section and compatibility with Texture and Color Enhancement Imaging (TXI™), Red Dichromatic Imaging (RDI™) and Narrow Band Imaging™ (NBI™) technologies when connected to the EVIS X1 CV-1500 video system center.
The ErgoGrip™ control section of EVIS X1 endoscopes is 10% lighter than the 190 series scope control section and features a rounded handle and easy-to-reach angulation control knobs and switches to accommodate users with small hands and support scope maneuverability.2
“As a leading global MedTech company, Olympus remains committed to providing advanced options to help physicians offer their patients the best care possible,” said Kurt Heine, Senior Vice President and General Manager for Gastrointestinal Solutions at Olympus Corporation. “Our goal is to elevate the standard of endoscopy, and Extended Depth of Field (EDOF) technology represents Olympus’ most advanced scope technology to help physicians drive the best clinical outcomes for advancing gastrointestinal procedures.”
The EVIS X1™ endoscopy system revolutionizes the way gastrointestinal disorders are detected, characterized and treated with innovative, easy-to-use diagnostic and therapeutic technologies and improved scope handling. The EVIS X1 endoscopy system provides a high level of patient care for every endoscopist, in every procedure, every day.
In addition to the newest EDOF technology, features of the EVIS X1 endoscopy system include:
- TXI™ technology, which is designed to increase the visibility of lesions and polyps by enhancing image color and texture.3
- RDI™ technology, which is designed to enhance the visibility of deep blood vessels and bleeding points.3
- NBI™ technology, which enhances visual observation of mucosal and vascular patterns by utilizing specific blue and green wavelengths absorbed by hemoglobin.3
- Brightness Adjustment Imaging with Maintenance of Contrast (BAI-MAC™) technology, which is designed to correct the brightness levels in dark areas of the endoscopic image, while maintaining the brightness of lighter areas, to increase the total distance view.4
TXI, RDI, BAI-MAC and NBI technologies are not intended to replace histopathological sampling as a means of diagnosis. These are adjunctive tools for endoscopic examination that can be used to supplement Olympus® white light imaging.
Visit the EDOF product page and the EVIS X1 product page or the gastroenterology page for more information about the entire Olympus GI portfolio.
About Olympus Corporation of the Americas
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states.
Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America. For more information, visit olympusamerica.com.
1 Data on file with Olympus (DC00493386, DC00433276, DC00510434 and DC00567392)
2 Data on file with Olympus (DC00600786, DC00482729, DC00031984, DC00482747, DC00779274, DC00031984, DC00600794, DC00481878, DC00031984, DC00841888 and DC00833645)
3 Data on file with Olympus (DC00489968)
4 Data on file with Olympus (DC00436067)
Download
* Products or devices presented include future technology which may be pending regional regulatory approval and are not available for sale in all regions.
* The contents in this website including products availability, specifications or prices are the information as of the date of announcement and are subject to change without prior notice.
* Information is intended to be presented to the media, shareholders, investors, and other interested parties. Information about our medical products (including products currently under development) included in this website is not intended for advertising or medical advice.
* Olympus Corporation assumes no responsibility for any damage resulting from the use of this material.
* All company names and product names mentioned in this website are trademarks or registered trademarks of their respective companies. ® and TM marks are not specified in this website. All trademarks and registered trademarks are the property of their respective owners.

